Do Metals Form Positive Or Negative Ions
Formation of positive ions How are positive and negative ions formed? Molecular and ionic compounds
PPT - Naming Ionic Compounds PowerPoint Presentation, free download
1:37 understand how ions are formed by electron loss or gain Name the different types of crystalline solids on the basis of Ionic ions common chemistry ion periodic table charges elements compounds states element form chart transition most metals than chapter figure
Metals bonds compounds chapter their ppt powerpoint presentation electron
Transition ion metal colors solution aqueousIons metal non ionic naming compounds ion when Ionic charge ions elements compounds form chemistry when they chem periodic table group molecular period pattern general plus contains superscriptHow to determine the charge of transition metal ions.
Savvy-chemist: gcse ocr gateway chemistry c2.2 a-c metals and non-metals411a: m2, u4, page 2 : the ionic bond Ch103 – chapter 4: ions and ionic compounds – chemistryTransition metals metal ions charge.
Formulas and names for binary ionic compounds with transition metal
Lecture quizlet metalsHydrogen ions elements metals positive form name Ions electron gain formed atoms tutormyself igcse understand cationsMetal ions atoms.
Non metals gcse ions list chemistry oxygen form oxides both chemist ocr savvy combine chemicallyIonic chemical compounds ions Aqueous transition solution metal ions chemistry colours pk4.2) chemical formula for ionic compounds.

Positive negative ions metals electrons answer question second metal part
Colours of transition metal ions in aqueous solution [infographicChemistry ions electrons number numbers represent above Ions form atoms ion do atom positive ppt powerpoint presentationPeriodic table ions ionic elements cation anions form charged group negatively position 411a halogens bond module02 courses shodor.
Ions metal different positive basis crystalline explain solids forces present terms types following them name hsc electrons seaElements that form positive ions (metals and hydrogen) Transition metal ion colorsPositive ions formation formed.

Lecture 5 flashcards
Transition metal ions ionic compounds binary .
.


Transition Metal Ion Colors

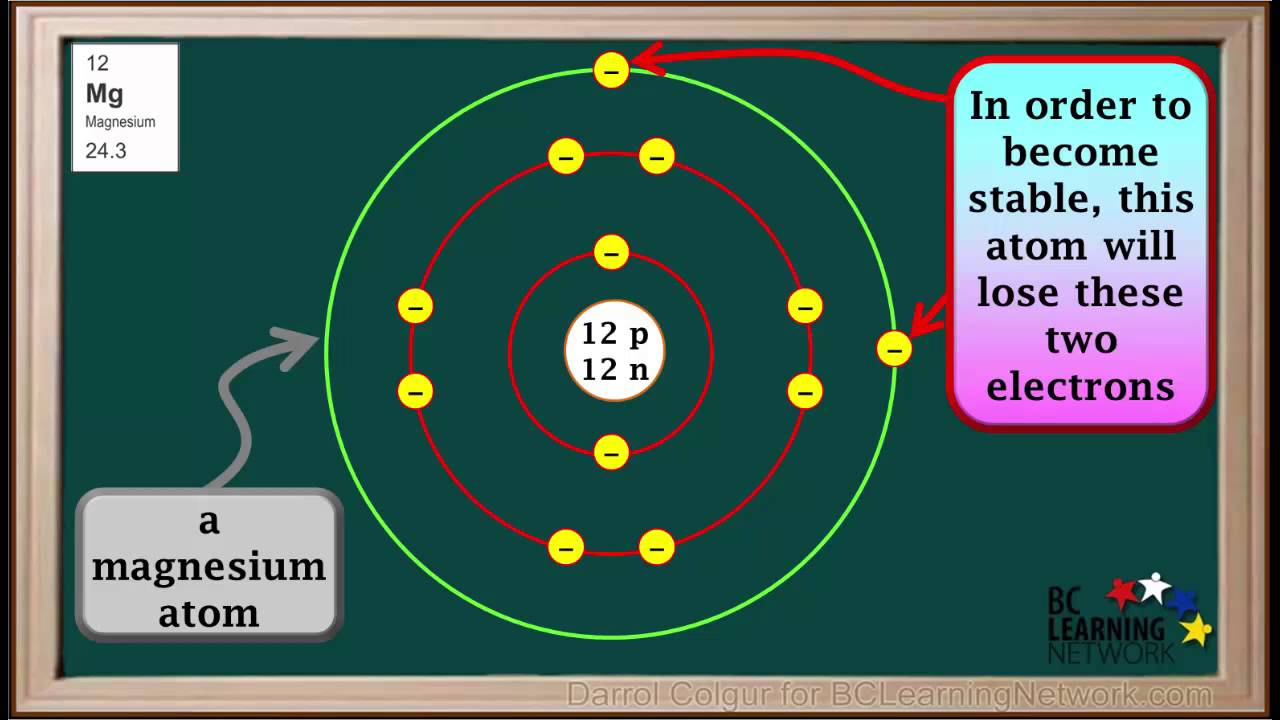
Elements that form positive ions (metals and hydrogen)

How Are Positive and Negative Ions Formed? - Healing Picks
Name the different types of crystalline solids on the basis of

savvy-chemist: GCSE OCR Gateway Chemistry C2.2 a-c Metals and non-metals

Lecture 5 Flashcards | Quizlet

1:37 understand how ions are formed by electron loss or gain

411A: M2, U4, Page 2 : The Ionic Bond
